There has been a wide divergence of opinion over the fate of Geronimo the Alpaca, as there is always likely to be following the death of an apparently healthy animal. This has led to some extremely negative press concerning TB testing in camelids. In forming an opinion about TB testing in camelids, it is important that the correct facts are known. I have seen a worrying amount of poor quality information appearing on social media and media channels over the last couple of months or so regarding bovine TB in camelids. So here is a little fact-busting on the issues.

TB Testing Data from DEFRA – and what it means
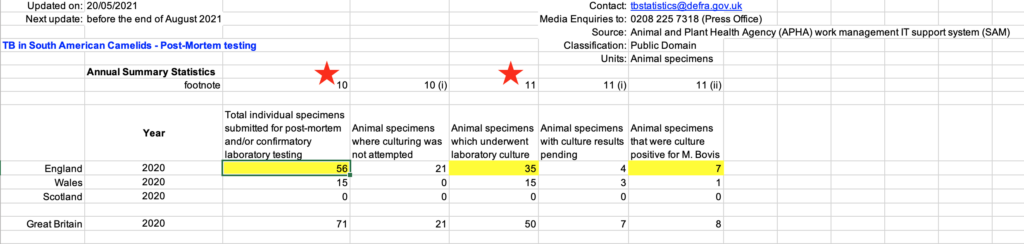
It has been stated by a group of vets in an open letter, the contents of which were published in the Vet Times, that “in 2020, 199 camelids were killed following a positive diagnosis by skin or blood tests”.1 The vets went on to say that “of 56 submitted for postmortem examination, only 4 had postmortem evidence of bTB, and successful culture of bTB was only achieved in 7 of the 35 animals where this was attempted.” This data was referenced as having been acquired from DEFRA’s Statistics on TB in non-bovine species which is a publication freely available on DEFRA’s website.2 They quote numbers for England only. Subsequently, on the 8th September at a protest outside DEFRA HQ, one of the signatories of the letter, vet Iain McGill, stated that “the specificity rather than being 99% (as quoted by DEFRA) was only 20%” and that “80% are false positives”. To understand where this came from, he has reached this conclusion by taking the number of culture positive cases (7) and dividing by the total number undergoing culture and represented this as the specificity of the test (7/35 x100 = 20%). However, this calculation is incorrect because he has misinterpreted what the data in the DEFRA tables means.
- The 35 specimens that underwent culture are from any camelids that had samples submitted to the APHA lab for culture. This will have included samples from the 56 camelids that were culled in England following a positive skin or blood test (“reactor” camelids), but also samples from animals that may have been suspect clinical cases or found to have some lesions at a routine PM.
- Seven specimens were positive on culture by the end of the year, while 4 culture results were still pending. This number is out of the 35 specimens that were submitted for culture, and this includes animals for which the diagnosis of TB was in doubt (eg cases of clinical suspicion). It is important to bear in mind that when a breakdown is confirmed by culture, very often further samples are not submitted from that herd for culture.
- Therefore, it CANNOT be concluded that only 7 of 35 reactor animals cultured positive for TB because the 35 animals were not all reactor camelids. Even if they were, the calculation would actually be demonstrating “positive predictive value” rather than specificity. Positive predictive value shows the probability that animals with positive test results actually have disease.3 We can’t actually calculate specificity from the figures presented because in order to do so, we would need to know the number of test positive animals that are actually NOT diseased (false positives), as well as the number of test negatives that truly do NOT have disease (true negatives). The DEFRA figures do not actually show this information because they are biased towards only those animals that tested positive for disease. In addition, Iain McGill is making an assumption that animals that did not have lesions were not infected. It is known that animals can be infected with TB despite not having any lesions. It is possible to prove an animal has TB, by finding of lesions and culturing mycobacteria, but it is much more difficult to prove a false positive – that is, an animal that clearly wasn’t even infected despite testing positive.
How good is testing in breakdown herds?
The data showing what proportion of the camelids that tested positive using an ante-mortem test, either skin test or serological test, had lesions at PM is not actually presented in DEFRA’s non-bovine statistics. Therefore it cannot be concluded that the antemortem testing performed was poorly sensitive at detecting disease. I personally have access to a reliable subset of these animals because 156 of the culled reactor camelids were on my family’s farm. A total of 113 of these alpacas were subjected to postmortem examination (PME) either by APHA (n=26) or myself (n=87), with help from colleagues. Therefore most of this does not appear in the DEFRA statistics. We performed the additional PMEs because we wanted to see for ourselves what proportion of reactors had lesions and specifically where those lesions were. We believe that at previous large herd breakdowns much valuable information was lost by disposal of carcases without PM. If lung lesions were present, we considered that they would be far more likely to spread TB to other camelids via spitting behaviour and feeding at shared troughs. We used this information to help control on-farm spread of TB.
Of the 13 skin test reactors post-mortemed, 10 had visible lesions. Of the 100 serological reactors post-mortemed, 70 had visible lesions. This gives us a positive predictive value within this single farm breakdown during 2020 of 70.1% overall. For serology alone it is 70% and for skin testing alone it is 77%. Positive predictive value is the probability that animals with a positive screening test result actually have the condition of interest. In the context of TB, this is based here purely on the presence or absence of lesions and excludes the possibility that some animals that do not have lesions may still in fact be infected or have been exposed.
What are typical lesions of TB in alpacas?
It has also been stated by certain vets that Geronimo did not show “typical lesions of TB” found in alpacas. Meanwhile, DEFRA vets were quoted clearly as stating that “a number of TB-like lesions were found in the left prescapular lymph node, mesenteric lymph node and liver, and they are indicative of TB”. Vets engaged by Geronimo’s owner, stated that he had “no white or cream caseous, enlarged abscesses typical for bTB in alpacas, whether in the lungs, bronchial, mediastinal or retropharyngeal lymph nodes.” Based on my experience performing postmortem examinations in alpacas affected with clinical TB, that is those that are experiencing clinical signs associated with infection with TB, you would find significant lesions in the lungs. It is fairly clear from the many photographs and videos available online that Geronimo did not have clinical pulmonary TB. TB can infect almost any organ in the body, including the liver, spleen, kidneys and abdominal lymph nodes. Lesions in the liver are extremely frequent in affected camelids. Hepatic TB (liver TB) may reflect infection via the intestinal tract due to ingestion of mycobacteria (rather than inhalation), or the lesions may have spread in the bloodstream from the lungs. In animals with lesions in the lungs, liver lesions may occur via either route, with mycobacteria in sputum being swallowed or spread in the bloodstream. Therefore not all TB infections result in lung lesions and liver lesions may be found in isolation. In my experience of performing postmortem examinations on serology reactors from a breakdown, liver lesions and those affecting the hepatic and mesenteric lymph nodes are extremely frequent and are often found without lesions in the lungs. These individuals are typically identified by ante-mortem skin and blood testing.
TB Testing for surveillance purposes
The justification for using these live animal tests to screen a herd is to identify infected individuals that may pose a risk to others in the herd: while they may not die from the disease, they may harbour latent infections that may pose a risk later. Some animals may indeed properly fight off the infection completely and not harbour live bacteria, but it is impossible to identify which ones have managed to do this. Even in humans the technology is not available. In humans it has been estimated that 25-30% of the world’s population are infected with TB and at risk of developing clinical TB – but this assumes that infection is life-long. Some have suggested that in fact only about 10% of those testing positive to immunological tests might still be infected, and have the potential to develop TB, and that tests to differentiate the infected from those with immunological memory of past infection (but not at risk) are vital.4 These tests would be highly valuable to us in the veterinary world too.
Serological testing of camelids has been shown to have approximately 97% specificity and 67% sensitivity.5 This means that around 33% of infected animals will fail to be detected by the test (false negative) while only about 3% of uninfected animals are incorrectly tested positive (false positive). This was based on finding lesions or not at PM. The Enferplex test is frequently used for voluntary testing of camelids: this test uses 7 different antigens to detect antibodies to TB. The more antigens that are positive on the test, the more likely the tested animal actually has the disease. When using the Enferplex test, the specificity can be improved to 99.7% when using the 4 antigen cut-off (instead of the 2 antigen cut-off), while the sensitivity drops to around 55%. The lower sensitivity of the screening test means that it is better to test more animals at one herd screening because if disease is present in the herd you are more likely to detect it. Imagine a fisherman throwing a net to catch a shoal of fish: you’re basically casting the net, knowing that you’ll miss some, but the bigger the shoal, the more likely you are to catch the ones you want. Any 2 antigen positive animals are given a retesting opportunity to give them the benefit of the doubt of the 3% chance of false positive at this level of interpretation.
“Priming” – or boosting of the antibody response using the skin test
Camelid owners are concerned about the possible effects of boosting the humoral response (often called “priming”) on increasing the incidence of false positive test results. While priming has been shown to be beneficial in breakdown herds at removing more potentially infected animals,6 it has not been tested properly in a screening situation. In practice, while few owners perform primed serological testing due to the additional costs involved, it is employed most commonly for statutory contiguous testing of camelids that fall within a 1km zone of a breakdown herd (usually cattle) in the high risk area for TB. This testing has resulted in the culling of a number of camelids that have subsequently been shown not to have lesions at PM. While this doesn’t necessarily prove that the animals were not infected, it highlights the need for research in this area especially since many of the camelids culled under these circumstances are pet animals and their removal together with the long wait to hear results has a significant emotional impact on their owners.
Thoughts on voluntary TB testing of camelids
Some in the camelid industry have suggested a boycott of voluntary testing of camelids. I would not support this as I see this as counterproductive and potentially harmful to the industry. Voluntary testing is used as a means of reducing the risk of spreading TB around the country from farm to farm. People doing voluntary testing for the main part use the Enferplex test alone (unprimed). If an animal tests positive at 2 Ag level it is permitted a retest under legislation and if found at the subsequent test to be negative (less than 2 Ag), it is assumed to have been a false positive and no further action is taken. If the retest shows increasing antibody titres, the likelihood of actual disease increases and animals are supposed to be removed. So testing allows for the possibility of potential false positives. If an animal is removed and found to have lesions, it may have detected a problem early that could save many more alpacas. Due to the relatively low sensitivity of serological testing, without whole herd testing, it is easier to miss infections in herds.
Unless you have experienced a TB breakdown, you have no idea how truly devastating this disease is in alpacas. Currently, people that do not test their animals pre-movement are risking passing this horrific disease on to unsuspecting buyers. I’m working with a herd currently that experienced their first TB death on their badger-proof fenced farm only 6 weeks after taking delivery of their animals from a breeder who said that testing for TB was unnecessary. The seller had also failed to post-mortem an animal a few months earlier that could have been the index case. This has been a horrendous experience for the buyer obviously as they are still shut down a year later and lost all of their potential stud males.
I have a proposal to investigate the issue of priming in alpacas and I hope to be able to get funding to proceed with this. Further research to study bovine TB in camelids in terms of evaluating the wide array of testing methods currently available in experimentally infected animals as well as the pathology of the disease and genetic susceptibility of camelids would also be extremely useful. It is my recommendation that funding sources are identified by the camelid industry to pursue these goals as the camelid industry itself has the most to gain from these endeavours.
This document is designed to provide some context to some of the information that has been put out into the public domain, and to provide some clarity. I have tried to explain some of the data that DEFRA have provided and to provide some new data to give an idea of what is actually going on. I need to be clear that there are deficiencies and problems with regard to TB policy concerning camelids that need to be addressed and that I have not highlighted here. Bovine TB is a big problem in the UK, and one that requires considerable focus and adaptability as we learn more and as new potential tests become available. All affected species need to be considered and the disease managed appropriately in all areas. Interaction with stakeholders to understand their concerns and address them properly is critical towards bringing the disease under control.
References
1. Silverwood J. Vets pen open letter on Geronimo case. In: Vet Times2021.
2. TB in non bovine species for 2011 to quarter 4 2020. In: DEFRA, ed. https://www.gov.uk/government/statistical-data-sets/other-tb-statistics: 2021.
3. Trevethan R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front Public Health 2017;5:307.
4. Behr MA, H EP, Ramakrishnan L. Is Mycobacterium tuberculosis infection life long? The British Medical Journal 2019;367.
5. Rhodes S, Holder T, Clifford D, et al. Evaluation of gamma interferon and antibody tuberculosis tests in alpacas. Clin Vaccine Immunol 2012;19:1677-1683.
6. Bezos J, Casal C, Alvarez J, et al. Evaluation of the performance of cellular and serological diagnostic tests for the diagnosis of tuberculosis in an alpaca (Vicugna pacos) herd naturally infected with Mycobacterium bovis. Prev Vet Med 2013;111:304-313.
